Integrated Rate Law For Second Order Reaction Pdf
Findings policy and intent a findings.
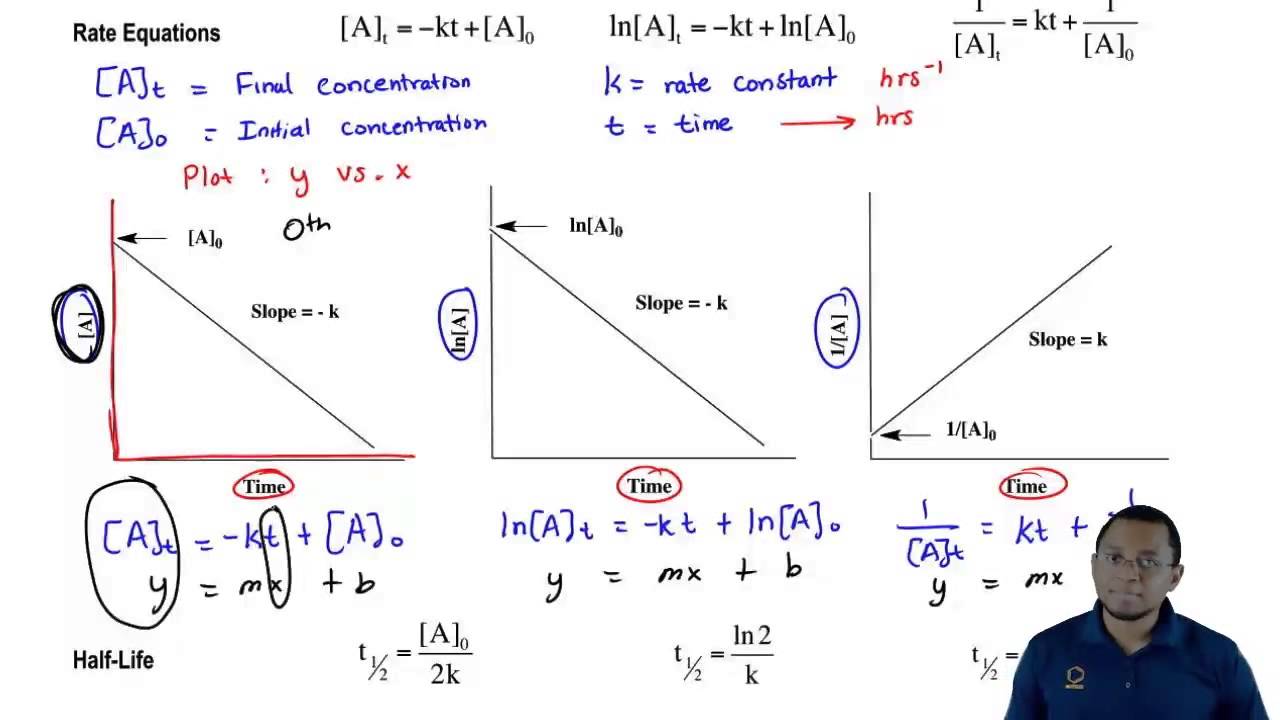
Integrated rate law for second order reaction pdf. Solving integrated rate law problems using the graphing calculator mt. Whitney high school ap chemistry the old fashioned and tedious method for determining the. The rate law or rate equation for a chemical reaction is an equation that links the reaction rate with the concentrations or pressures of the reactants and constant. The reaction rate or rate of reaction is the speed at which reactants are converted into products.
For example the oxidative rusting of iron under earths atmosphere. 1 chapter 12 chemical kinetics. 121 reaction rates. Study of the speed with which reactants are converted to products.
Box and cox 1964 developed the transformation. Estimation of any box cox parameters is by maximum likelihood. Box and cox 1964 offered an example in which the. Our staff cant provide legal advice interpret the law or conduct research.
You may be able to obtain assistance from a lawyer or paralegal. It is important to note that the light intensity which is proportional to the rate of reaction increases to a peak before declining with time. Subchapter icontrol of toxic substances 2601.